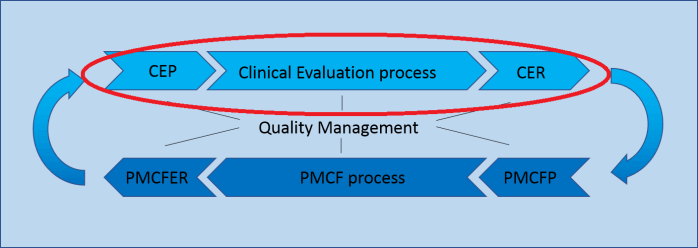
Clinical Evaluation (CER)
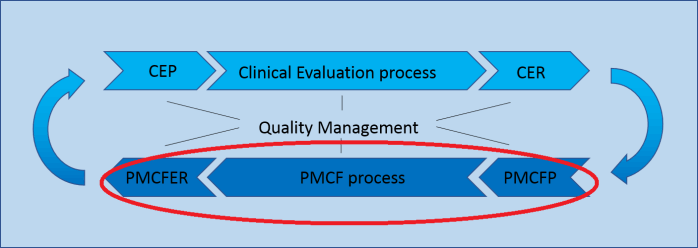
Post-Market Clinical Follow-up (PMCF)
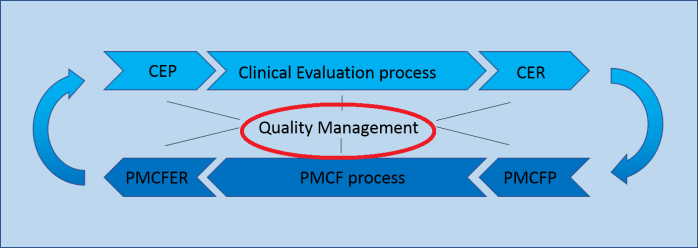
Quality Management
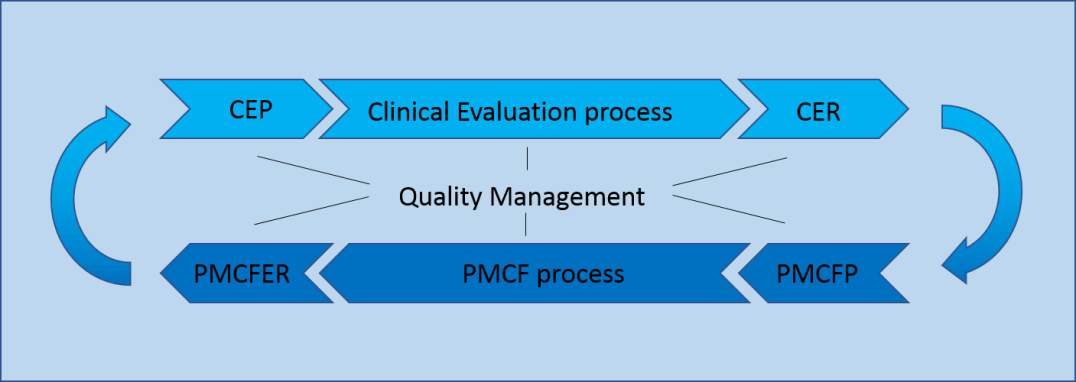
Clinical Evaluation (CER) and Post-Market Clinical Follow-up (PMCF) are closely related
According to the European Medical Device Regulation (MDR), manufacturers have to provide clinical data for their medical devices after they have been placed on the market.
The planning and implementation effort for suitable follow-up measures (PMCF) is increasing, not only for medical devices that are to be newly CE-certified, but also for medical devices for which re-certification is pending.
Manufacturers should develop PMCF plans that include all PMCF activities for the expected life cycle of their devices.